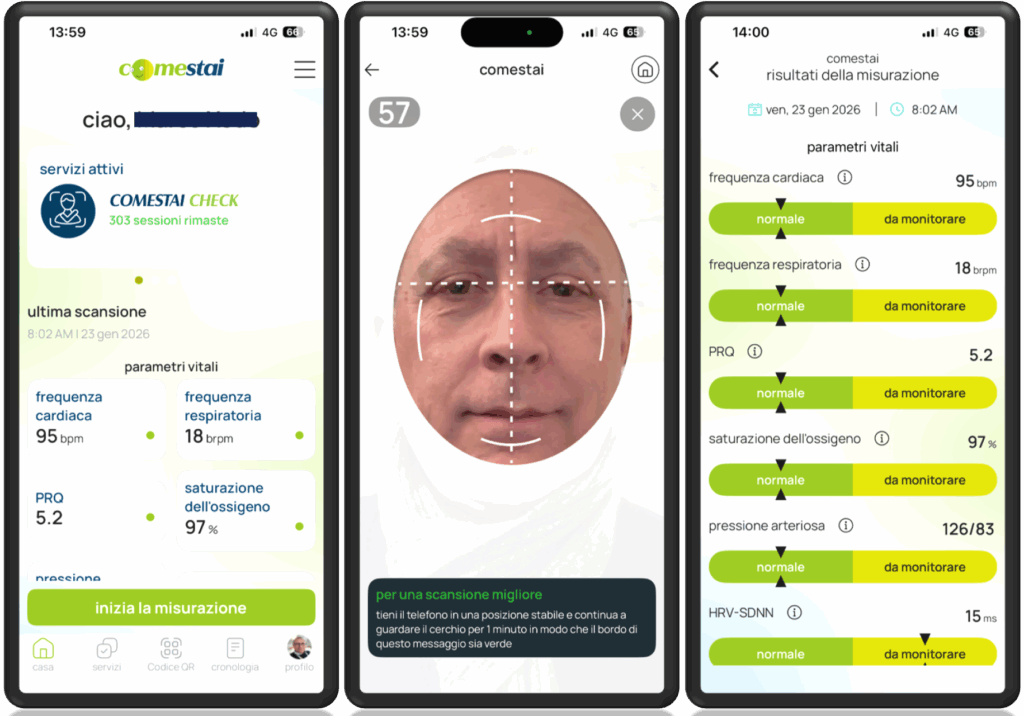
Comestai Italy, a digital health company that builds an end-to-end ecosystem for mass prevention, having at its core comestai check, the first smartphone-only, software medical device (CE Class I under EU MDR) that allows users to measure multiple vital and risk parameters in one minute using only a phone camera, and a strategic partner of Binah.ai, the world’s leading provider of AI-powered video-based health and wellness monitoring solutions, today announced a major milestone in the evolution of remote health monitoring and preventive health: the company has officially received CE Medical Device Regulation (MDR) Class 1 certification CE: Reg. al MdS R/2741800 for its comprehensive mobile app, web-based platform and SDK powered by Binah SDK (Software Development Kit).
This certification, combined with new GDPR-compliant cloud storage options in Italy, establishes the combined Comestai and Binah.ai solutions as the most flexible and secure gateway for European enterprises.
The MDR Class 1 certified solutions are ideally suited for the insurance and pharmaceutical sectors, in use cases such as underwriting, health trends and risks monitoring, telemedicine, travel insurance, triage and many others.
Certified Security, Minimal Liability
By achieving MDR Class 1, Comestai provides a certified framework for companies to monitor vital signs through any camera-equipped device. Because the solution is designated for health monitoring rather than clinical diagnosis and requires end-user consent of personal health data, corporate clients can integrate these tools with the assurance of a medically recognized standard while minimizing the liability issues often associated with non-certified health software.
The Most Comprehensive Solution in Europe
With this announcement, Binah.ai and Comestai Italy offer the European market three distinct, high-flexibility paths to leverage Binah.ai’s technology:
- Standalone comestai App: Deploy the Comestai App as a ready-to-use, standalone solution for immediate market entry. White label services bundle can also be developed (CE Medical Device Regulation (MDR) Class 1).
- Web Integration: Use the co-branded Comestai Web to seamlessly integrate health monitoring capabilities into existing web platforms and ecosystems. (CE Medical Device Regulation (MDR) Class 1).
- Custom Development: Utilize the comestai SDK to build a fully customizable, cobranded app. (CE Medical Device Regulation (MDR) Class 1). The third-party app needs to be certified.
- Custom Development: Utilize the Binah SDK to build a fully customizable, branded app tailored for general wellness, for use cases that do not require medical certification*.
Localized Data Sovereignty (GDPR)
Addressing the strict data privacy requirements of the European Union, Comestai has obtained GDPR certification for its Italian-based cloud infrastructure. This provides European companies with three robust options for data management:
- On-Device Storage: Keep all health data strictly on the user’s local device.
- Client Cloud: Store data on the client’s own cloud (subject to the client’s GDPR certification).
- Comestai Cloud: Leverage Comestai’s certified Italian cloud for a fully managed, compliant data storage solution.
A Solution for Enterprise Leaders
“Our goal is to eliminate barriers to digital health adoption in Europe,” said Paolo Osvaldo Agnelli, CEO at Come Stai S.p.a.. “By combining CE MDR Class I certification with localized, GDPR-compliant cloud options, we are offering insurance companies and pharmaceutical leaders a ‘plug-and-play’ ecosystem that is both medically sound and legally secure.”, continues Agnelli.
“Our strategic partnership with comestai delivers exactly what the European market has been waiting for: a medically certified, GDPR-compliant bridge to the future of health and wellness,” said David Maman, Co-founder and CEO of Binah.ai. The comestai and Binah.ai partnership is ideally suited for large-scale deployments in Life and Health Insurance (for digital underwriting and policyholder engagement, travel insurance), Pharmaceuticals (for patient recruitment and monitoring in clinical trials), and Corporate Wellness programs.
For more information or to request a trial of the Come Stai–Binah.ai solutions, please reach out to [email protected] or [email protected].
About Come Stai
Come Stai S.p.a. is a premier provider of digital health solutions, specializing in bridging the gap between cutting-edge AI technology and regulatory compliance. Based in Italy, Come Stai empowers enterprises to deliver secure, certified, and user-friendly health monitoring tools.
About Binah.ai
Binah.ai’s award-winning, software-based solution transforms camera-equipped devices – like a smartphone or tablet – into a health monitoring tool. Our technology extracts a wide range of vital signs and health indicators, including bloodless blood tests, taken via a simple 35-50-second video of a person’s face. Bridging health gaps by blending technology, science, and humanity, our solution serves over 150 companies globally in the insurance, healthcare, and wellness industries. By enabling real time objective risk assessment, effective remote patient monitoring, and data-driven wellness programs, we help businesses positively impact the well-being of millions of people, making health monitoring accessible to everyone, everywhere.
*Note: Binah.ai’s product is for general wellness use only and is not intended to diagnose, treat, cure, or prevent any medical condition.



